Cellular and Molecular Engineering
The field of cellular and molecular engineering explores the mechanical, electrical, and chemical behavior of cells and biomolecules with the ultimate goal of improving health. Engineers work toward achieving this goal through the analysis of molecular interactions and signaling, the theoretical modeling of cellular systems, the design of novel interacting molecules, and the development of innovative detection technologies and devices. The bioengineering department displays a strong research background in this field as demonstrated by the works of Dr. William Hancock, professor of bioengineering; Dr. Cheng Dong, distinguished professor of bioengineering; and Dr. Peter Butler, associate professor of bioengineering. These researchers have made significant impact in the scientific community through their fundamental works and integrated research strategies.
The Molecular Biomechanics laboratory in bioengineering is directed by Dr. Hancock. His overreaching philosophy involves “getting deep into the fundamental biological questions with an engineering perspective … to think quantitatively using applied physics, applied chemistry, and analytical and computational modeling.”
His work focuses on the fundamental biophysics of kinesin motor proteins and applications involving kinesins for biotechnology and microscale engineering. Kinesins are involved in many facets of cell biology, including axonal transport in neurons, mitosis, and organelle and virus transport. This functional transport system also holds the potential as a tool for bioseparations and sensors.
One main project in Dr. Hancock’s lab investigates the fundamental aspects of Kinesin‑2 motors through techniques such as motility assays and stopped-flow analysis. He utilizes modeling as tool to interpret data and gain insight into molecular mechanisms. Through the study of specific kinesin behavior, this project aims to identify commonalities between motors and improve knowledge of general motor mechanisms.
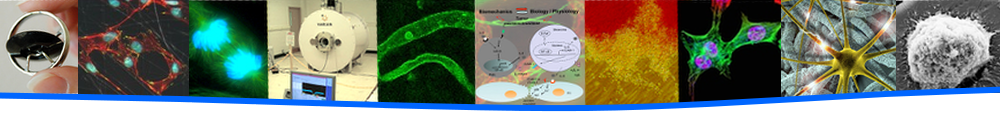
Model of the microtubule steering mechanism (top) and image of microtubule at right colliding with static microtubule and being bent by fluorescently-labeled EB1-kinesin complex (bottom).
Another project studies microtubule polarity in neurons, focusing on the maintenance of minus-end out geometry in dendrite microtubules. Through the work of Dr. Melissa Rolls, assistant professor of biochemistry and molecular biology, and her lab at Penn State, a model for microtubule steering at junctions was developed involving the formation of an EB1-kinesin-2 complex. In this model, the complex bends the microtubule tip toward the cell body upon encounter with a static microtubule, to maintain minus-end out orientation in dendrites (shown in the image below). Dr. Hancock’s lab investigates this model by reconstituting the EB1-Kinesin-2 complex to visualize this bending phenomenon and use computational modeling to understand the biophysics and mechanics of the process.
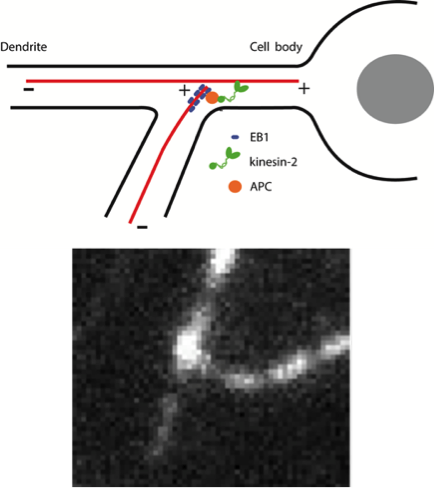
Studies on the cell signaling and protein dynamics between cancer and immune cells through the analysis of cell adhesion and motility under flow conditions.
The Cellular Mechanics Laboratory in bioengineering is led by Dr. Dong and focuses on how the tumor microenvironment alters immune cell functions. His work has evolved to answer important questions concerning how cell-cell and cell-protein signaling affect cellular functions, deformation, adhesion and motility within the circulation during cancer metastasis. Further, his novel development of a cell extravasation apparatus allows innovative studies of active cell adhesion to and transmigration through the endothelium under dynamic flow conditions.
A current research project involves how the tumor microenvironment changes the endothelia gap junction biology, specifically focusing on how tumor cell adhesion to the endothelium would induce junction disassembly. Dr. Dong is collaborating with Dr. Esther Gomez, assistant professor of chemical engineering and bioengineering, to investigate the mechanism of gap formation in response to the tumor microenvironment.
Another project involves systems network modeling of the signal pathways within endothelial cells to elicit gap junction formation in response to tumor cell signals. Using protein reaction kinetics, these simulations can identify pathways that are most sensitive to signal inputs. Experimental studies can be used in parallel to validate these simulations.
Dr. Dong also works with Dr. Jim Adair, professor of materials science and engineering and bioengineering, to effectively deliver drugs to circulating tumor cells. His motivation stems from the question, “how can you actively deliver an inhibitor or drug to cells as you would treat them on the bench top?”
He answers the question by encapsulating the drugs in a nanocarrier that is targeted to cells in vivo with the same efficiency as bench top studies. His work involves the characterization of nanocarrier targeting and binding to tumor cells.
The Cellular Mechanobiology Laboratory in bioengineering, headed by Dr. Butler, currently focuses on single-molecule techniques to better understand mechanobiology. Dr. Butler is interested in the dynamic nature of the cellular membrane and notes that, unlike the term ‘biomechanics’, “‘mechanobiology’ seems to be more focused on biological processes and how mechanics influence them.”
Dr. Butler believes that the mechanics of a cell may affect its dynamics and organization as well as its shape and flexibility. With this concept in mind, his research has diversified from cell biology and mechanics to single molecule spectroscopy and its applications in nanoscale drug delivery vehicles.
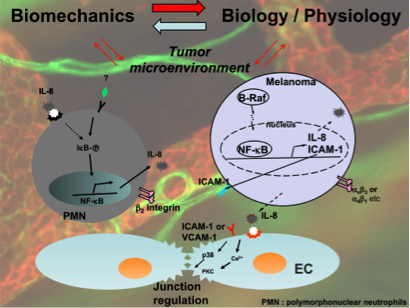
Optical trap surface rheometry enables detection of adhesion events.
Dr. Butler also has a project involving the organization of the cell membrane, specifically the dynamics of cell attachment and interaction with surfaces. Cells can attach to surfaces through focal adhesions. He has developed a technique using a pipette with feedback control to touch a cell and observe how molecules organize around this adhesion. He has also used optical traps to detect the moment of adhesion and measure its force.
Another study stems from a technique that Dr. Butler has developed to measure stress in membranes using fluorescence. The stress measurement relies on the area per lipid, which, he has observed, fluctuates in cells. With these measurements, the tension in membranes may be determined. Further, Dr. Butler has been able to correlate membrane tension with the uptake of nanoparticles by the cell. In these studies, he has found that high membrane tension prevents nanoparticle uptake, while low tension permits this uptake. This study, a collaboration with Dr. Sulin Zhang, associate professor of engineering science and mechanics and bioengineering, combines fundamental physics with an application that is expected to impact future research in mechanobiology and drug delivery.
- Erin Richards, Ph.D. Candidate